Many recent articles have called for overhauling antibiotic pricing as one of the key ways to lure investments in antibiotic development. But, its beneficial effects, in my opinion, are exaggerated.
Alarm bells are being rung with several articles over the last few weeks in the Wall Street Journal, New York Times, and The Washington Post (to name a few) discussing how challenging the climate is for antibiotic developers. Many quoted in these articles call for an overhaul of antibiotic pricing with the central thesis being that such an overhaul will provide much needed revenue and attract investment for struggling antibiotic developers, thus recharging the efforts to combat the antimicrobial crisis. But, will it?
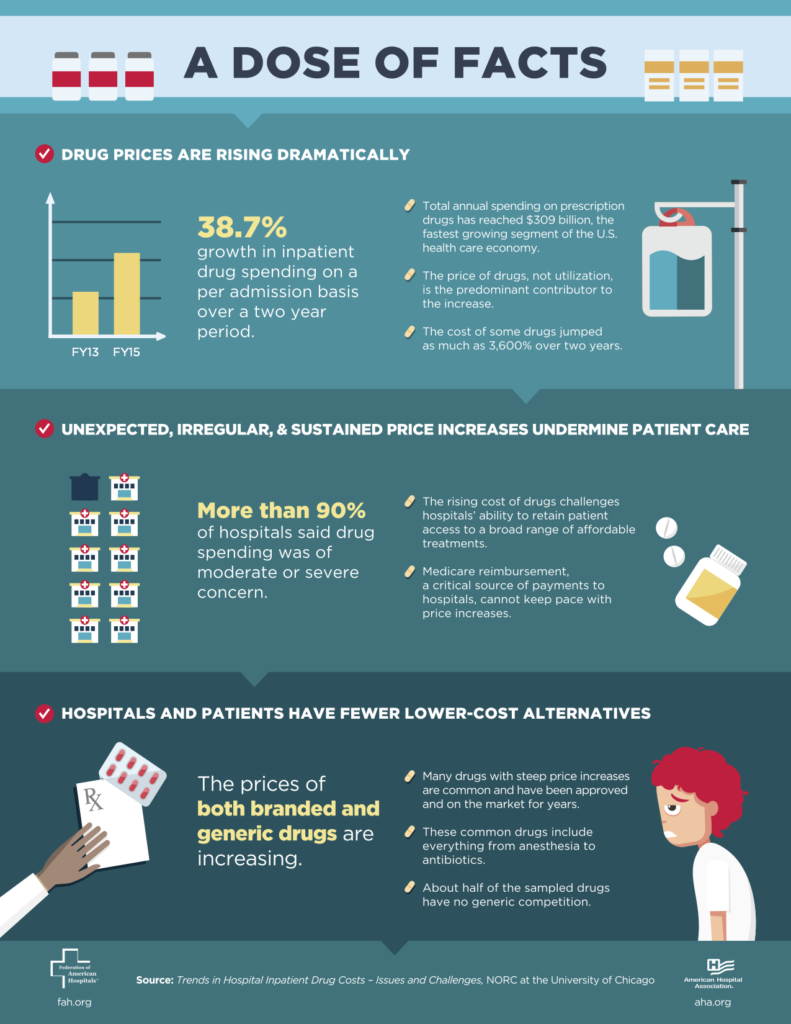
Past efforts to increase drug prices generated a lot of opposition and much hand wringing (see infographic). Those objections were however generally directed towards very expensive cancer drugs or overpricing of drugs. But the fundamental reasons for the opposition have not disappeared.
Certainly, antibiotic developers are struggling. They do not earn anywhere close to the revenue that a successful cancer therapeutic developer would and these struggles are well documented. But what will increasing the price/reimbursement of an antibiotic really do? The thinking goes that if antibiotic developers are successful, it will draw in more money and talent creating a virtual cycle that will be self-sustaining.
But, is it self-sustaining and will it achieve its desired goals? Everyone acknowledges that the two key reasons why antibiotic developers struggle are because (a) hospitals prescribe these new drugs sparingly to avoid building up resistance to them and (b) they try a lower cost alternative first.
When decisions on which antibiotic to use are still based entirely on clinical judgment, i.e. a good educated guess, going with the lower cost option or hoarding a “drug of last resort” is logical and natural. Why do we think that increasing reimbursement will alter this behavior. And unless we do something that changes this dynamic we are not really solving the problem. We are just creating a new special interest.
Antibiotics are the lifeblood of modern medicine. And antimicrobial resistance is a global problem. If the price of the antibiotic were raised, will other countries break patents invoking “national emergencies” much as was done to get inexpensive HIV drugs?
If the drugs are inexpensive in, say India, and are used extensively there because they work, how long do you think it will be before resistance develops for these drugs?
If you went to a physician and after a visual examination, the physician recommends that you undergo cancer treatment because he/she suspects you have cancer, would you do it or would you change physicians? But, this is what we do every single day with infections and antibiotics. And if someone comes along saying the solution is to have pricier drugs, well…
Look, I get the point about reviewing pricing of antibiotics to aid discovery and development of new drugs. They need help. But, these can be one-time efforts. But, overhauling reimbursements of drugs (which sets up a long-term impact) is not the answer to combating antimicrobial resistance. It is not sustainable and will do nothing to change prescribing behaviors. When we can assure appropriate usage of antibiotics, we can influence prescription behavior and then revenues will take care of themselves.
Aha! So how do we assure appropriate usage of antibiotics? The only way I am aware of is by having a diagnostic that can truly guide treatment decisions along with improved awareness and preventive measures that hospitals are already beginning to take. If such a test were available, then physicians would not hoard. They would not try the lower cost option just because it is there. But they will pick the drug because it will provide maximum benefit to the patient. And because we will not be overusing or misusing the antibiotic, resistance spread will be slower. Note that all of these benefits can be achieved without raising drug pricing. So I would say that we should be redoubling efforts to ensure appropriate usage of antibiotics (especially new antibiotics) that meet the needs of hospitals, physicians, and patients and not fiddling with drug pricing.
You might say my position is biased because of my affiliation. It is not. But, the best way to counter my argument would be to provide a better self-sustaining model – one that helps developers, patients, and the healthcare system. Overhauling pricing helps the developers directly and very tenuously, the patients, healthcare system, or society. And as much as I relate with drug developers (having been one), such a strategy (increasing pricing/reimbursements) does not make for good policy.
